
Chemistry, 10.03.2020 08:21 lovebunny33921
CO(g)+2H2(g)⇌CH3OH(g)CO(g)+2H2(g)⇌C H3OH(g) This reaction is carried out at a different temperature with initial concentrations of [CO]=0.27M[CO]=0.27M and [H2]=0.49M[H2]=0.49M. At equilibrium, the concentration of CH3OHCH3OH is 0.11 MM. Find the equilibrium constant at this temperature.

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 12:40
In the following table, all the columns for the element calcium are filled out correctly. element electron structure of atom electron structure of ion net ionic charge calcium 1s22s22p63s23p64s2 1s32s22p63s23p64s1 +1 true false
Answers: 2

Chemistry, 22.06.2019 14:00
Will mark brainliest how many electrons can be held in the energy level n = 4?
Answers: 1


Chemistry, 23.06.2019 01:30
Magnesium is the limiting reactant in this experiment. calculate the theoretical yield of mgo for each trial. trial 1: trial 2: data mass of empty crucible with lid trial 1: 26.688 trial 2: 26.681 mass of mg metal, crucible, and lid trial 1: 26.994 trial: 2 26.985 mass of mgo, crucible, and lid trial 1: 27.188 trial 2: 27.180
Answers: 1
You know the right answer?
CO(g)+2H2(g)⇌CH3OH(g)CO(g)+2H2(g)⇌C H3OH(g) This reaction is carried out at a different temperature...
Questions

English, 17.12.2020 16:10

English, 17.12.2020 16:10

Mathematics, 17.12.2020 16:10

Mathematics, 17.12.2020 16:10




Mathematics, 17.12.2020 16:10

Mathematics, 17.12.2020 16:10

Physics, 17.12.2020 16:10

Mathematics, 17.12.2020 16:10

English, 17.12.2020 16:10

Biology, 17.12.2020 16:10


Chemistry, 17.12.2020 16:10

Mathematics, 17.12.2020 16:10




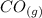 +
+ 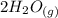 ⇄
⇄ 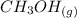
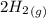 ⇄
⇄ ![K_C = \frac{[CH_3OH]}{[CO][H_2]^2}](/tpl/images/0540/6074/3667b.png)
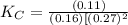
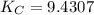
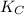 = 9.4
= 9.4

