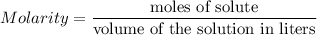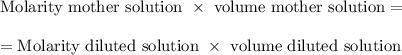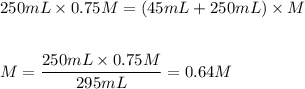Chemistry, 10.03.2020 08:20 emmv565628
If 45 mL of water are added to 250 mL of a 0.75 M K2SO4 solution, what will the molarity of the diluted solution be?

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 05:40
Calculate: select the worksheet tab. this tab you calculate the analyte concentration. fill in the first set of boxes ("moles h2so4" and "moles naoh") based on the coefficients in the balanced equation. (if there is no coefficient, the value is 1.) record the appropriate volumes in the "ml naoh" and "ml h2so4" boxes. record the concentration of the titrant in the m naoh box. click calculate. what is the concentration listed
Answers: 2


Chemistry, 23.06.2019 01:00
What is the chemical name of the compound ti2o3? use the list of polyatomic ions and the periodic table to you answer.
Answers: 1

Chemistry, 23.06.2019 01:10
Volume is a measurement of how fast particles of a substance are moving
Answers: 3
You know the right answer?
If 45 mL of water are added to 250 mL of a 0.75 M K2SO4 solution, what will the molarity of the dilu...
Questions

Mathematics, 31.03.2021 17:40



English, 31.03.2021 17:40

Mathematics, 31.03.2021 17:40

Social Studies, 31.03.2021 17:40

Mathematics, 31.03.2021 17:40


English, 31.03.2021 17:40

Mathematics, 31.03.2021 17:40

Mathematics, 31.03.2021 17:40

Mathematics, 31.03.2021 17:40

Arts, 31.03.2021 17:40


Biology, 31.03.2021 17:40

Social Studies, 31.03.2021 17:40



History, 31.03.2021 17:40

Mathematics, 31.03.2021 17:40