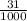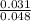Chemistry, 10.03.2020 08:16 jordandabrat
1) You have an aqueous solution where [OH-] = 1 x 10-4 mol/L.
A. Calculate the hydrogen ion concentration [H+]. Show all work.
B. Calculate the pH. Show all work.
C. Is this solution an acid, base, or neutral? You must explain how you know.
2) Calculate the molarity of a solution prepared by dissolving 195 g of sodium chloride (NaCl) in enough water to make 2.8 liters of solution.
3) A student takes 152 mL of a 3.0M HCl solution and places it into a beaker. The student then adds enough water to the beaker to make 750 mL. Calculate the molarity of this new solution. Show all work.
4) A student started a titration by placing 20.0 mL of 0.24 M NaOH into a flask with 3 drops of phenolphthalein. The student then titrated to the endpoint using 31.0 mL of HCl. Calculate the concentration (molarity) of the HCl that the student used. Show all work for credit.

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 05:00
Given sno2 + 2h2 - sn + 2h20 tin oxide reacts with hydrogen to produce tin and water. how many moles of sno2 are needed to produce 500.0 grams of sn?
Answers: 3

Chemistry, 22.06.2019 23:00
In which region is the substance in both the solid phase and the liquid phase? 1 2. 3 4 mark this and return save and exit next
Answers: 2

Chemistry, 23.06.2019 02:00
Calculate the molarity of each aqueous solution: a. 78.0 ml of 0.240 m naoh diluted to 0.250 l with water b. 38.5 ml of 1.2 m hno3 diluted to 0.130 l with water
Answers: 1

You know the right answer?
1) You have an aqueous solution where [OH-] = 1 x 10-4 mol/L.
A. Calculate the hydrogen ion co...
A. Calculate the hydrogen ion co...
Questions



Social Studies, 25.03.2021 01:00

Mathematics, 25.03.2021 01:00

Mathematics, 25.03.2021 01:00


Mathematics, 25.03.2021 01:00

Mathematics, 25.03.2021 01:00

Health, 25.03.2021 01:00



English, 25.03.2021 01:00

Mathematics, 25.03.2021 01:00


Mathematics, 25.03.2021 01:00

Mathematics, 25.03.2021 01:00


Mathematics, 25.03.2021 01:00


Mathematics, 25.03.2021 01:00

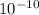 x is the hydrogen ion concentration.
x is the hydrogen ion concentration.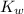 = [
= [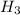 O+] [OH]-
O+] [OH]- 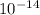
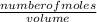
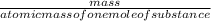
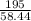 ( atomic weight of NaCl is 58.44 gm/mole)
( atomic weight of NaCl is 58.44 gm/mole)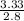
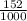 = M2 ×
= M2 × 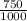
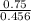
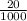 = Macid x
= Macid x