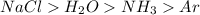Chemistry, 10.03.2020 08:25 darius12318
Arrange the molecules H2O, NH3, Ar, NaCl in order of expected increasing boiling points. 1. None of these 2. NaCl, H2O, NH3, Ar 3. Ar, NH3, H2O, NaCl 4. NH3, Ar, H2O, NaCl

Answers: 3


Another question on Chemistry

Chemistry, 21.06.2019 22:10
What does a particular point on a line of a phase diagram represent? o a. the maximum temperature a substance can exist at without bonds breaking b. the pressure created by the kinetic energy of molecules at a particular temperature c. the melting point or boiling point of a substance at a specific pressure d. the conditions in which temperature and pressure have equal effects on a substance
Answers: 2


Chemistry, 22.06.2019 02:30
Which element forms an ionic bond with flourine? 1) fluorine 2) carbon 3) potassium 4) oxygen
Answers: 1

Chemistry, 22.06.2019 05:30
You are making a solution of calcium chloride dissolved in water. you add solid, stir, and it dissolves. you add just a spatula tip full, stir, and the solid does not dissolve. how could you describe the solutions before and after adding the spatula tip amount
Answers: 1
You know the right answer?
Arrange the molecules H2O, NH3, Ar, NaCl in order of expected increasing boiling points. 1. None of...
Questions


Mathematics, 13.07.2021 02:20

Biology, 13.07.2021 02:20

Mathematics, 13.07.2021 02:20


Social Studies, 13.07.2021 02:20

Chemistry, 13.07.2021 02:20


Mathematics, 13.07.2021 02:20


Mathematics, 13.07.2021 02:20

Engineering, 13.07.2021 02:20

History, 13.07.2021 02:20

Engineering, 13.07.2021 02:20

Health, 13.07.2021 02:20



Mathematics, 13.07.2021 02:20

Physics, 13.07.2021 02:20

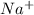 and
and 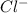 ions are held together by strong coloumbic attractive force. Hence NaCl has highest boiling point'Ar is a monoatomic molecule. Hence only weak london dispersion force exists between Ar atom/molecules. Hence it requires lowest amount of energy to boil and thereby possess lowest boiling point.
ions are held together by strong coloumbic attractive force. Hence NaCl has highest boiling point'Ar is a monoatomic molecule. Hence only weak london dispersion force exists between Ar atom/molecules. Hence it requires lowest amount of energy to boil and thereby possess lowest boiling point.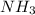 and
and 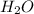 are polar protic molecules. Hence they possess london dispersion force, dipole-dipole force and hydrogen bonding as intermolecular forces.Dipole-dipole force is stronger in
are polar protic molecules. Hence they possess london dispersion force, dipole-dipole force and hydrogen bonding as intermolecular forces.Dipole-dipole force is stronger in