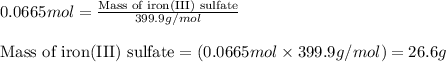Chemistry, 10.03.2020 08:01 jocelynm0611
The chemical equation shows iron(III) phosphate reacting with sodium sulfate. 2FePO4 + 3Na2SO4 Fe2(SO4)3 + 2Na3PO4 What is the theoretical yield of Fe2(SO4)3 if 20.00 g of FePO4 reacts with an excess of Na2SO4

Answers: 1


Another question on Chemistry

Chemistry, 20.06.2019 18:04
According to the balanced chemical equation 5 h2c2o4(aq) + 2 mno4-(aq) + 6 h+(aq) → 10 co2(g) + 2 mn2+(aq) + 8 h2o(l) 0.875 grams of oxalic acid, h2c2o4 will react with moles of permanganate, mno4-.
Answers: 2

Chemistry, 22.06.2019 01:00
Which statement correctly describes potassium iodide, ki? there is a one-to-one ratio of potassium ions to iodide ions. potassium gains electrons and iodine loses electrons during the reaction. the lattice is held together by potassium anions and iodide cations.
Answers: 1

Chemistry, 22.06.2019 05:50
What are transitions between a liquid and gas called? identify which way they are transitioning
Answers: 2

You know the right answer?
The chemical equation shows iron(III) phosphate reacting with sodium sulfate. 2FePO4 + 3Na2SO4 Fe2(S...
Questions


Computers and Technology, 16.03.2020 20:00




English, 16.03.2020 20:00




English, 16.03.2020 20:00

English, 16.03.2020 20:00


Computers and Technology, 16.03.2020 20:00


Computers and Technology, 16.03.2020 20:00



Arts, 16.03.2020 20:00

Social Studies, 16.03.2020 20:01

Social Studies, 16.03.2020 20:01

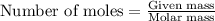 .....(1)
.....(1)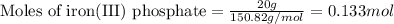
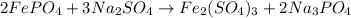
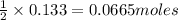 of iron(III) sulfate
of iron(III) sulfate