Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 10:00
Americium-241 undergoes fission to produce three neutrons per fission event. if a neutron-absorbing material is mixed in with this sample so that the rate of neutron production drops down to 1.8 neutrons per fission event, which will be effective at achieving a critical mass? check all that apply. remove a deflective shield surrounding the sample. remove absorbent material mixed in with the sample. compress the sample of americium-241.
Answers: 1

Chemistry, 22.06.2019 10:00
Ill give brainiestif one neutron initiates a fission event that produces two neutrons in the products, how many new reactions can now be initiated? if each of the neutrons produced in the first fission event then initiates a fission event that produces one neutron in the products, how many new reactions can now be initiated by each neutron? how many neutrons in total were produced by the two fission events described?
Answers: 2

Chemistry, 22.06.2019 22:30
What must be in balance for temperatures to remain constant?
Answers: 1

Chemistry, 22.06.2019 23:00
What is the oxidation state of each individual carbon atom in c2o42−?
Answers: 1
You know the right answer?
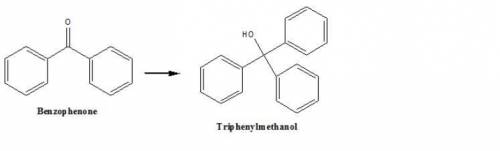
This reaction forms triphenylmethanol from benzophenone. How could you use infrared spectroscopy to...
Questions

History, 21.10.2020 02:01


Mathematics, 21.10.2020 02:01



Biology, 21.10.2020 02:01




Mathematics, 21.10.2020 02:01


Mathematics, 21.10.2020 02:01



Mathematics, 21.10.2020 02:01

Mathematics, 21.10.2020 02:01




English, 21.10.2020 02:01

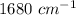 and frequency band of this wavelength is absent in triphenylmethanol.
and frequency band of this wavelength is absent in triphenylmethanol. 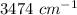 and frequency band of this wavelength is absent in benzophenone.
and frequency band of this wavelength is absent in benzophenone.