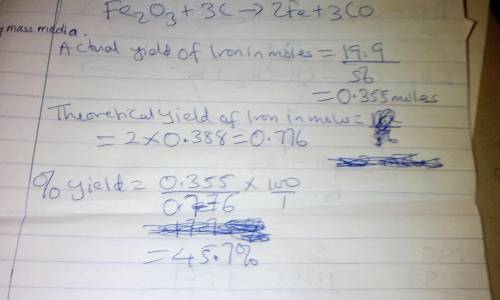Chemistry, 10.03.2020 08:05 quanwalker651370
Combining 0.338 mol Fe 2 O 3 with excess carbon produced 19.9 g Fe . Fe 2 O 3 + 3 C ⟶ 2 Fe + 3 CO What is the actual yield of iron in moles? actual yield: mol What is the theoretical yield of iron in moles? theoretical yield: mol What is the percent yield? percent yield:

Answers: 1


Another question on Chemistry

Chemistry, 21.06.2019 22:30
200. ml of 3.00 m nacl solution is diluted to a final volume of 500. ml. what is the molarity of the final solution?
Answers: 2

Chemistry, 22.06.2019 08:00
Joe shines white light into a bowl half full of water at an angle of incident of 27.5°. calculate the angle of refraction in the water given the indices of refraction for air and water are 1.00 and 1.36, respectively.
Answers: 2

Chemistry, 22.06.2019 08:30
If i initially have a gas at a pressure of 12 atm, a volume of 23 liters, and a temperature of 200 k, and then i raise the pressure to 14 atm and increase the temperature to 300 k, what is the new volume of the gas?
Answers: 1

Chemistry, 22.06.2019 21:00
One similarity and one difference between an element and a mixture of elements
Answers: 1
You know the right answer?
Combining 0.338 mol Fe 2 O 3 with excess carbon produced 19.9 g Fe . Fe 2 O 3 + 3 C ⟶ 2 Fe + 3 CO Wh...
Questions







Computers and Technology, 16.10.2019 02:00


Biology, 16.10.2019 02:00



Mathematics, 16.10.2019 02:00

Mathematics, 16.10.2019 02:00


Biology, 16.10.2019 02:00



English, 16.10.2019 02:00