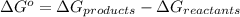For the following reaction, label each of the below species as an acid or a base. Use lower case letters only (e. g. acid)
HCN + HPO4⁻² ⇔ H2PO4⁻ + CN⁻
Will the above reaction take place spontaneously? (Is the reaction product-favored? Does the equilibrium lie to the right?)

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 02:00
In the following redox reaction which is the oxidizing agent and which is the reducing agent? alcl3 + na nacl + al
Answers: 1


Chemistry, 22.06.2019 12:10
Consider the reaction: n2(g) + o2(g) ⇄ 2no(g) kc = 0.10 at 2000oc starting with initial concentrations of 0.040 mol/l of n2 and 0.040 mol/l of o2, calculate the equilibrium concentration of no in mol/l how would this be done?
Answers: 3

Chemistry, 22.06.2019 15:20
Select the most likely product for this reaction: koh(aq) + co2(g) – ? k2co3(aq) + h2o(1) k(s) + h2(g) + o2(g) k(s) + co3(9) +h2
Answers: 2
You know the right answer?
For the following reaction, label each of the below species as an acid or a base. Use lower case let...
Questions

Chemistry, 23.03.2020 23:45

Mathematics, 23.03.2020 23:45

Mathematics, 23.03.2020 23:45

Biology, 23.03.2020 23:45


Mathematics, 23.03.2020 23:45


Mathematics, 23.03.2020 23:45


Mathematics, 23.03.2020 23:45


Mathematics, 23.03.2020 23:46


Mathematics, 23.03.2020 23:46

Computers and Technology, 23.03.2020 23:46




Health, 23.03.2020 23:46

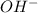 .
. 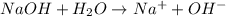
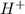 .
.
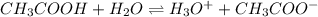
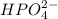 is accepting the hydrogen ions so it acts as a base.
is accepting the hydrogen ions so it acts as a base.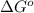 values of the given species are as follows.
values of the given species are as follows.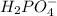 = -1130.4 kJ/mol,
= -1130.4 kJ/mol, 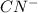 = 172.4 kJ/mol
= 172.4 kJ/mol