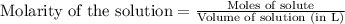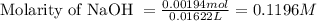A 0.397-g sample of potassium hydrogen phthalate, KHC8H4O4 (molar mass = 204.22 g/mol) is dissolved with 50 mL of deionized water in a 125-mL Erlenmeyer flask. The sample is titrated to the phenolphthalein endpoint with 16.22 mL of a sodium hydroxide solution. What is the molar concentration of the NaOH solution? (Show all calculations)

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 04:00
What three natural resources are found in the great lakes region
Answers: 2

Chemistry, 22.06.2019 16:50
What is conserved in the reaction shown below? h2(g) + cl2 (g) --> 2hcl(g)a. mass onlyb. mass and moles onlyc. mass, moles, and molecules onlyd. mass, moles, molecules, and volume
Answers: 2

Chemistry, 22.06.2019 21:40
A5 mole sample of liquid acetone is converted to a gas at 75.0°c. if 628 j are required to raise the temperature of the liquid to the boiling point, 15.600 kj are required to evaporate the liquid, and 712 j are required to raise the final temperature to 75.0°c, what is the total energy required for the conversion?
Answers: 3

Chemistry, 22.06.2019 23:00
Which of your 24 wells had indications that a chemical reaction occurred? how were you able to tell that a chemical reaction occurred? which of your 24 wells had indications that a physical reaction occurred? how were you able to tell that a physical reaction occurred? report on both mixing and evaporation. make a general statement about whether your hypotheses were validated or rejected. must your hypotheses be correct for this to be a successful laboratory?
Answers: 3
You know the right answer?
A 0.397-g sample of potassium hydrogen phthalate, KHC8H4O4 (molar mass = 204.22 g/mol) is dissolved...
Questions

Mathematics, 24.02.2021 23:50

Physics, 24.02.2021 23:50




History, 24.02.2021 23:50

Mathematics, 24.02.2021 23:50


Mathematics, 24.02.2021 23:50

Biology, 24.02.2021 23:50

Mathematics, 24.02.2021 23:50




Mathematics, 24.02.2021 23:50

Mathematics, 24.02.2021 23:50

Chemistry, 24.02.2021 23:50

Mathematics, 24.02.2021 23:50


English, 24.02.2021 23:50

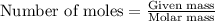
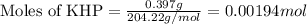
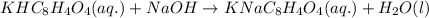
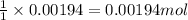 of NaOH.
of NaOH.