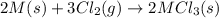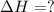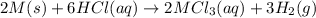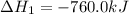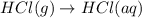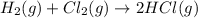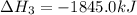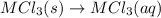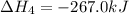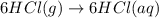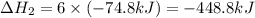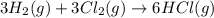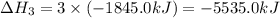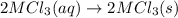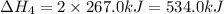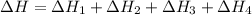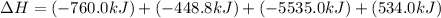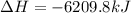Chemistry, 10.03.2020 09:08 greeneashlynt
Consider these reactions, where M represents a generic metal. 2 M ( s ) + 6 HCl ( aq ) ⟶ 2 MCl 3 ( aq ) + 3 H 2 ( g ) Δ H 1 = − 760.0 k J HCl ( g ) ⟶ HCl ( aq ) Δ H 2 = − 74.8 k J H 2 ( g ) + Cl 2 ( g ) ⟶ 2 HCl ( g ) Δ H 3 = − 1845.0 k J MCl 3 ( s ) ⟶ MCl 3 ( aq ) Δ H 4 = − 267.0 k J Use the given information to determine the enthalpy of the reaction 2 M ( s ) + 3 Cl 2 ( g ) ⟶ 2 MCl 3 ( s )

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 09:00
Suppose you have designed a new thermometer called the x thermometer. on the x scale the boiling point of water is 129 ? x and the freezing point of water is 13 ? x. part a at what temperature are the readings on the fahrenheit and x thermometers the same?
Answers: 1

Chemistry, 22.06.2019 12:30
Nebulae are enormous clouds in outer space. they are made mostly of hydrogen gas, helium gas, and dust. some nebulae glow brightly, while others do not. the stars that people see are huge, bright balls of glowing gas. they are made mostly of hydrogen and helium. which statement correctly describes other ways in which nebulae and stars are different? a. stars can form inside a nebula but a nebula can never be produced by any star. b. a star always has a higher density than a nebula. c. stars can never form inside a nebula but a nebula can be produced by any star. d. a nebula always has a higher density than a star.
Answers: 3

Chemistry, 22.06.2019 17:40
If 3 moles of a compound use 24 j of energy in a reaction, what is the a hreaction in j/mol?
Answers: 1

Chemistry, 22.06.2019 22:30
Amedication is given at a dosage of 3.000 mg of medication per kg of body weight. if 0.1500 g of medication is given, then what was the patient's weight in pounds (lbs)? there are 453.59g in 1 lb.
Answers: 2
You know the right answer?
Consider these reactions, where M represents a generic metal. 2 M ( s ) + 6 HCl ( aq ) ⟶ 2 MCl 3 ( a...
Questions


English, 24.03.2020 19:13





History, 24.03.2020 19:14






Social Studies, 24.03.2020 19:15

Mathematics, 24.03.2020 19:15


Mathematics, 24.03.2020 19:16




Mathematics, 24.03.2020 19:16