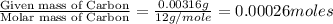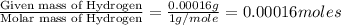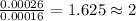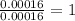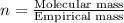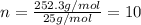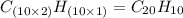Chemistry, 10.03.2020 09:06 auviannadority13
One of the components of natural crude oil and coal deposits is benzo[a]pyrene, a compound with a molecular mass of about 252.3 amu, containing only carbon and hydrogen. A 3.320 mg sample of benzo[a]pyrene burns to give 11.58 mg of CO2. Determine its empirical and molecular formulas. (Omit states-of-matter from your answer.)

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 04:50
Acompound contains c, h, and o atoms. when 1.130 g of the compound is burned in oxygen, 1.064 g co2 and 0.3631 g h2o are produced. what is the empirical formula of this compound?
Answers: 1

Chemistry, 22.06.2019 08:40
Write the formula for the following chemicals. 7. e. trinitrogen tetraoxide a calcium phosphate f. magnesium acetate b. potassium sulfide g nickel(iii) cyanide c carbon dioxide h. silver sulfate d. cobalt(ii) chloride
Answers: 1

Chemistry, 22.06.2019 09:00
Which process does not require the presence of a physical substance in order to transfer heat? air in the atmosphere is heated by the ground. this warm air then rises, and cooler air falls. this is an example of what type of process? how is conduction different from radiation?
Answers: 1

Chemistry, 22.06.2019 13:30
An animal cell loses the ability to convert energy stored in food to energy that the cell can use. which of the cell's organelles has stopped working? a.the mitochondria b.the nucleus c.the vacuoles d.the endoplasmic reticulum
Answers: 1
You know the right answer?
One of the components of natural crude oil and coal deposits is benzo[a]pyrene, a compound with a mo...
Questions



Computers and Technology, 18.11.2020 23:10

English, 18.11.2020 23:10

English, 18.11.2020 23:10

Mathematics, 18.11.2020 23:10









Mathematics, 18.11.2020 23:10

Biology, 18.11.2020 23:10



SAT, 18.11.2020 23:10

History, 18.11.2020 23:10

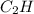 and
and 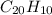
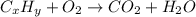
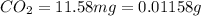 (Conversion factor: 1 g = 1000 mg)
(Conversion factor: 1 g = 1000 mg)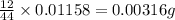 of carbon will be contained.
of carbon will be contained.