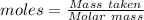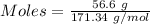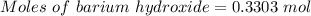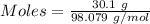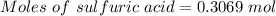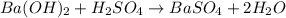For the following reaction, 56.6 grams of barium hydroxide are allowed to react with 30.1 grams of sulfuric acid. barium hydroxide (aq) + sulfuric acid (aq) barium sulfate (s) + water (l) What is the maximum amount of barium sulfate that can be formed? grams What is the FORMULA for the limiting reagent? What amount of the excess reagent remains after the reaction is complete? grams

Answers: 2


Another question on Chemistry

Chemistry, 21.06.2019 22:30
How much heat is released upon converting one mole of steam (18.0 g) from 100.0 ∘c to water at 25.0 ∘c? show work and constants, trying to figure out how it works. only given the heat capacity for steam and water so try to only use that
Answers: 1

Chemistry, 22.06.2019 08:40
Write the formula for the following chemicals. 7. e. trinitrogen tetraoxide a calcium phosphate f. magnesium acetate b. potassium sulfide g nickel(iii) cyanide c carbon dioxide h. silver sulfate d. cobalt(ii) chloride
Answers: 1

Chemistry, 23.06.2019 01:00
Which polymers are most closely related? a. protein and nucleic acids b. cellulose and starch c. nucleic acids and starch d. nucleic acids and cellulose
Answers: 2

Chemistry, 23.06.2019 09:30
Which of the following is not a characteristic of a hydrogen bond? 1. it is responsible for the unusual physical properties of water. 2. it is weaker than a covalent bond. 3. it is stronger than other dipole-dipole interactions. 4. it can occur when hydrogen is covalently bound to very electronegative elements liks f, cl, br and i.
Answers: 1
You know the right answer?
For the following reaction, 56.6 grams of barium hydroxide are allowed to react with 30.1 grams of s...
Questions

History, 05.05.2020 15:39

Mathematics, 05.05.2020 15:39


History, 05.05.2020 15:39

History, 05.05.2020 15:39

Mathematics, 05.05.2020 15:39



History, 05.05.2020 15:39

Mathematics, 05.05.2020 15:39


Chemistry, 05.05.2020 15:39

English, 05.05.2020 15:39

Mathematics, 05.05.2020 15:39

Law, 05.05.2020 15:39

Mathematics, 05.05.2020 15:39



Chemistry, 05.05.2020 15:39

 .
.