Chemistry, 10.03.2020 09:12 faithyholcomb
A 22.4 L vessel contains 0.02 mol H2 gas, 0.02 mol N2 gas, and 0.1 mol NH3 gas. The total pressure is 700 torr. What is the partial pressure of the H2 gas

Answers: 3


Another question on Chemistry

Chemistry, 21.06.2019 20:30
As you move from right to left on the periodic table the atomic radius fill in the blank
Answers: 2

Chemistry, 21.06.2019 22:30
Often on a topographic map, every fifth contour line is darkened. what is this line called? a. key b.slope c.benchmark d. index contour
Answers: 1

Chemistry, 22.06.2019 06:30
Melting and boiling are endothermic processes. this means that these processes absorb energy from their surroundings in order to occur. use this information and the data you collected in the phase change gizmo to describe what happens to the temperature of water when you boil it, then explain why this result occurs.
Answers: 1

Chemistry, 22.06.2019 12:10
Achemistry student needs to standardize a fresh solution of sodium hydroxide. he carefully weighs out of oxalic acid , a diprotic acid that can be purchased inexpensively in high purity, and dissolves it in of distilled water. the student then titrates the oxalic acid solution with his sodium hydroxide solution. when the titration reaches the equivalence point, the student finds he has used of sodium hydroxide solution.calculate the molarity of the student's sodium hydroxide solution. be sure your answer has the correct number of significant digits.
Answers: 1
You know the right answer?
A 22.4 L vessel contains 0.02 mol H2 gas, 0.02 mol N2 gas, and 0.1 mol NH3 gas. The total pressure i...
Questions


Physics, 03.07.2019 08:30

English, 03.07.2019 08:30

History, 03.07.2019 08:30


Physics, 03.07.2019 08:30


Mathematics, 03.07.2019 08:30

History, 03.07.2019 08:30


History, 03.07.2019 08:30



Chemistry, 03.07.2019 08:30


Mathematics, 03.07.2019 08:30

Mathematics, 03.07.2019 08:30



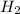 is 98 torr
is 98 torr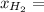
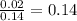
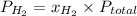
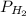 and
and 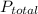 are partial pressure of
are partial pressure of