
Chemistry, 10.03.2020 09:01 devin030505
Problem PageQuestion Gaseous ethane will react with gaseous oxygen to produce gaseous carbon dioxide and gaseous water . Suppose 8.42 g of ethane is mixed with 48. g of oxygen. Calculate the minimum mass of ethane that could be left over by the chemical reaction. Round your answer to significant digits.

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 02:30
When svante arrhenius first proposed his acid-base theory, he was a doctoral candidate. his professors thought his ideas were unfounded. within a decade, the arrhenius theory of acid-base was widely accepted and praised within the scientific world. arrhenius defined acids as compounds having ionizable hydrogen and bases as compounds with ionizable a) barium. b) hydronium. c) hydroxide. d) oxygen.
Answers: 3

Chemistry, 22.06.2019 04:30
Electrons are extremely important to what area of technology? a) anti-aging research b) household product development c) electronics d) drug discovery
Answers: 3

Chemistry, 22.06.2019 06:40
Which statement correctly describes metallic bonds? a. they form when certain atoms lose electrons and other atoms gain electrons. b. they involve an attraction between anions and cations. they always involvpoth a metal and a nonmetal. d. they can only form between atoms of the same element. e. they form because electrons can move freely between atoms.
Answers: 3

Chemistry, 23.06.2019 07:00
If you used the method of initial rates to obtain the order for no2, predict what reaction rates you would measure in the beginning of the reaction for initial concentrations of 0.200 m, 0.100 m, & 0.050 m no2.
Answers: 3
You know the right answer?
Problem PageQuestion Gaseous ethane will react with gaseous oxygen to produce gaseous carbon dioxide...
Questions

Chemistry, 22.03.2021 17:50




Mathematics, 22.03.2021 17:50


Mathematics, 22.03.2021 17:50

Mathematics, 22.03.2021 17:50

Social Studies, 22.03.2021 17:50




Mathematics, 22.03.2021 17:50

Biology, 22.03.2021 17:50

Mathematics, 22.03.2021 17:50


Mathematics, 22.03.2021 17:50


Mathematics, 22.03.2021 17:50

Mathematics, 22.03.2021 17:50

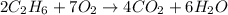
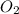 react completely with 2 moles of
react completely with 2 moles of 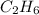
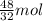 of
of 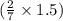 moles of
moles of 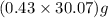 = 12.9 g
= 12.9 g

