

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 13:30
The atomic number, or number, is the described as the number of in the nucleus of an chemical element.
Answers: 1

Chemistry, 22.06.2019 20:00
For the reaction c6h14(g) & longrightarrow; c6h6(g) + 4h2(g), δp(h2)/δt was found to be 2.5 x 10-2 atm/s, where δp(h2) is the change in pressure of hydrogen. determine δp(c6h14)/δt for this reaction at the same time.
Answers: 2

Chemistry, 22.06.2019 22:50
At the current rate, a graph of carbon dioxide produced by fossil fuels over time would slope upward slope downward be horizontal be vertical
Answers: 3

You know the right answer?
Write the equation for the equilibrium that corresponds to each of the following reaction quotients....
Questions

Mathematics, 25.08.2021 04:50


Engineering, 25.08.2021 04:50

Mathematics, 25.08.2021 04:50

Physics, 25.08.2021 04:50

Biology, 25.08.2021 04:50


Mathematics, 25.08.2021 04:50





English, 25.08.2021 04:50

Advanced Placement (AP), 25.08.2021 04:50

Chemistry, 25.08.2021 04:50

Mathematics, 25.08.2021 04:50

English, 25.08.2021 04:50

Mathematics, 25.08.2021 04:50

Advanced Placement (AP), 25.08.2021 04:50

Mathematics, 25.08.2021 04:50

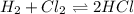
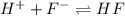
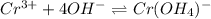
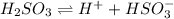
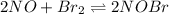
![Q=\frac {[HCl]^2]}{[H2][Cl2]}](/tpl/images/0540/8245/690ab.png)
![Q=\frac {[HF]}{[H^+][F^-]}](/tpl/images/0540/8245/729d4.png)
![Q=\frac {[Cr(OH_4)^-]}{[Cr^{3+}][OH^-]^4}](/tpl/images/0540/8245/d1a63.png)
![Q=\frac {[H^+][HSO_3^-]}{[H_2SO_3]}](/tpl/images/0540/8245/f5250.png)
![Q=\frac {[NOBr]^2}{[NO]^2 [Br_2]}](/tpl/images/0540/8245/ad7eb.png)


