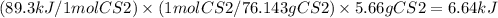Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 03:50
Consider the reaction: n2(g) + o2(g) ? 2no(g) kc = 0.10 at 2000oc starting with initial concentrations of 0.040 mol/l of n2 and 0.040 mol/l of o2, calculate the equilibrium concentration of no in mol/l how would this be done?
Answers: 3

Chemistry, 22.06.2019 15:30
Light waves can move through , but they travel fastest when they move through a(n) .
Answers: 1

Chemistry, 22.06.2019 23:00
What is the average rate of the reaction between 10 and 20 s?
Answers: 1

Chemistry, 23.06.2019 09:00
Describe the process that was used in this lab to create magnesium oxide, specifically identifying the type of chemical reaction. explain why the product had a higher mass than the reactant, and how this relates to conservation of matter.
Answers: 2
You know the right answer?
When carbon disulfide is formed from its elements, heat is absorbed. Calculate the amount of heat (i...
Questions


Mathematics, 03.04.2020 05:14

History, 03.04.2020 05:14

Mathematics, 03.04.2020 05:14





English, 03.04.2020 05:15

Mathematics, 03.04.2020 05:15

Mathematics, 03.04.2020 05:15

Mathematics, 03.04.2020 05:15






Social Studies, 03.04.2020 05:15

Health, 03.04.2020 05:15

Biology, 03.04.2020 05:15