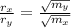Chemistry, 10.03.2020 18:02 ethangeibel007
How can comparing the rates of effusion of gases X and Y allow the molar mass of Y to be calculated? Write an equation stating the relationship between the ratio of the X and Y rates of effusion and their molar masses, MX and MY.

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 00:30
Jessica is traveling from miami, florida, to chicago, illinois. using the map, tell one way the land will change during the second half of her trip.
Answers: 1

Chemistry, 22.06.2019 02:00
If you add 10ml of hot water to 10ml of cold water and the change in tempature 8°c calculate how much energy is gained by the cold water
Answers: 1

Chemistry, 22.06.2019 05:50
Why doesn't heat added to water make the tempature rise above 100c
Answers: 2

Chemistry, 22.06.2019 11:40
Which of these expressions are correct variations of the combined gas law? p1v1t2 = p2v2t1 both
Answers: 2
You know the right answer?
How can comparing the rates of effusion of gases X and Y allow the molar mass of Y to be calculated?...
Questions

Mathematics, 18.11.2020 16:30

History, 18.11.2020 16:30





Arts, 18.11.2020 16:30


History, 18.11.2020 16:30


Mathematics, 18.11.2020 16:30





Chemistry, 18.11.2020 16:30

Social Studies, 18.11.2020 16:30



English, 18.11.2020 16:30