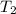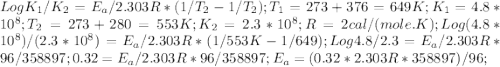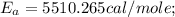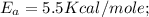The rate constant k for a certain reaction is measured at two different temperatures:
te...

Chemistry, 10.03.2020 18:49 kkwolfcityouwc96
The rate constant k for a certain reaction is measured at two different temperatures:
temperature k
376.0 °c 4.8 x 108
280.0 °C 2.3 x 10 8
Assuming the rate constant obeys the Arrhenius equation, calculate the activation energy Ea for this reaction.
Round your answer to 2 significant digits.

Answers: 2


Another question on Chemistry


Chemistry, 22.06.2019 08:30
In the millikan oil drop experiment they determined that every drop had a charge which was a while number multiple of -1.60x10^-19. if a drop has a total charge of -9.60x10^-19 then how many excess electrons are contained within the drop?
Answers: 2

Chemistry, 22.06.2019 19:20
Anyone who's in connections academy chemistry b have the factors that affect the rate of a reaction portfolio already done?
Answers: 3

Chemistry, 23.06.2019 01:00
Substance 33°f 100°f peanut oil solid liquid margarine solid liquid chocolate chips solid liquid which conclusion fits the data in the table? a. heat chemically changes chocolate and margarine. b. all solids become liquid at 100°f. c. removing heat from a substance it to melt. d. matter may change shape when it is heated.
Answers: 1
You know the right answer?
Questions



Physics, 02.06.2021 04:50

History, 02.06.2021 04:50


Mathematics, 02.06.2021 04:50

Mathematics, 02.06.2021 04:50



Chemistry, 02.06.2021 04:50

Biology, 02.06.2021 04:50



Mathematics, 02.06.2021 04:50

Mathematics, 02.06.2021 04:50

Biology, 02.06.2021 04:50

Chemistry, 02.06.2021 04:50

History, 02.06.2021 04:50


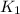 and
and 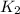 at temperature
at temperature 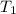 and
and