

Answers: 1


Another question on Chemistry



Chemistry, 22.06.2019 20:00
Suppose that some of the compound spilled out of the crucible after it was heated. would that cause the percent by mass of water in the compound determined by the experiment to be too low, too high, or unchanged? briefly explain your answer.
Answers: 1

Chemistry, 22.06.2019 21:30
Which of the following changes will decrease the total amount of gaseous solute able to be dissolved in a liter of liquid water? (2 points) decreasing temperature decreasing pressure decreasing surface area decreasing solute concentration
Answers: 1
You know the right answer?
Reaction rate is expressed in terms of changes in the concentration of reactants and products. Write...
Questions

English, 14.02.2021 14:00

Mathematics, 14.02.2021 14:00


Advanced Placement (AP), 14.02.2021 14:00


Computers and Technology, 14.02.2021 14:00

Biology, 14.02.2021 14:00

Mathematics, 14.02.2021 14:00

Mathematics, 14.02.2021 14:00





Biology, 14.02.2021 14:00


Social Studies, 14.02.2021 14:00



Mathematics, 14.02.2021 14:00

Mathematics, 14.02.2021 14:00

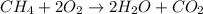
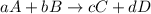
![\text{Rate of disappearance of A}=-\frac{1}{a}\frac{d[A]}{dt}](/tpl/images/0541/3019/2d8eb.png)
![\text{Rate of disappearance of B}=-\frac{1}{b}\frac{d[B]}{dt}](/tpl/images/0541/3019/1e77e.png)
![\text{Rate of formation of C}=+\frac{1}{c}\frac{d[C]}{dt}](/tpl/images/0541/3019/cee4b.png)
![\text{Rate of formation of D}=+\frac{1}{d}\frac{d[D]}{dt}](/tpl/images/0541/3019/7ef32.png)
![Rate=-\frac{1}{a}\frac{d[A]}{dt}=-\frac{1}{b}\frac{d[B]}{dt}=+\frac{1}{c}\frac{d[C]}{dt}=+\frac{1}{d}\frac{d[D]}{dt}](/tpl/images/0541/3019/d4b94.png)
![Rate=-\frac{d[CH_4]}{dt}=-\frac{1}{2}\frac{d[O_2]}{dt}=+\frac{1}{2}\frac{d[H_2O]}{dt}=+\frac{d[CO_2]}{dt}](/tpl/images/0541/3019/dfd60.png)


