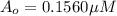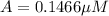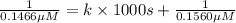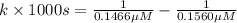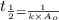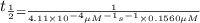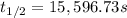Chemistry, 10.03.2020 19:03 leannaadrian
Nitrous acid (HNO2) slowly decomposes to NO, NO2, and water by the following second-order reaction: 2 HNO2(aq) ---> NO(g) + NO2(g) + H2O(l) Use the date below to determine the rate law and the constant for this reaction: Time (s) [HNO2] (um) 0 0.1560 1000 0.1466 1500 0.1424 2000 0.1383 2500 0.1345 3000 0.1309 A) Rate = ?? B) k = ??? /uM*s C) Determine the half-life for the decomposition of HNO2: t1/2 = ?

Answers: 1


Another question on Chemistry

Chemistry, 21.06.2019 17:00
Initially, the balloon had 3.0 liters of gas at a pressure of 400 kpa and was at a temperature of 294 k. if the balloon is cooled to 277 k and its volume decreased to 1 l, what will the new pressure in the balloon be?
Answers: 1

Chemistry, 22.06.2019 08:30
Which metal exist in liquid state and can be cut with knife ?
Answers: 2


Chemistry, 23.06.2019 05:00
Scientists discovered fossils in several layers of the earth you see here. they found fossils of algae, snails, and clams in layer d. given that information, where do you think they found fossil evidence of simple land plants and amphibians?
Answers: 1
You know the right answer?
Nitrous acid (HNO2) slowly decomposes to NO, NO2, and water by the following second-order reaction:...
Questions







Arts, 06.10.2021 21:40

Biology, 06.10.2021 21:40




Mathematics, 06.10.2021 21:40

English, 06.10.2021 21:40

World Languages, 06.10.2021 21:40



Mathematics, 06.10.2021 21:40



![R=k[HNO_2]^2](/tpl/images/0541/2623/c6302.png)
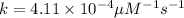
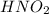 is 15,596.73 s
is 15,596.73 s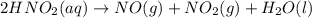
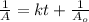
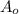 = initial concentration
= initial concentration