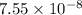Chemistry, 10.03.2020 19:01 topangabraith
Two equilibrium reactions of nitrogen with oxygen, with their corresponding equilibrium constants (Kc) at a certain temperature, are given below. reaction (1): N2(g) + O2(g) equilibrium reaction arrow 2 NO(g); Kc = 2.59e-31 reaction (2): N2(g) + 1/2 O2(g) equilibrium reaction arrow N2O(g); Kc = 3.31e-24 Using this set of data, determine the equilibrium constant for the following reaction, at the same temperature. reaction (3): N2O(g) + 1/2 O2(g) equilibrium reaction arrow 2 NO(g)

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 06:00
One does not belong why? ice, gold ,wood ,diamond and table salt
Answers: 1


Chemistry, 22.06.2019 20:30
Which states of matter have particles that move independently of one another with very little attraction?
Answers: 1

Chemistry, 22.06.2019 20:30
A40 kilogram skier starts at the top of a 12 meter high slope. at the bottom, she is travelling 10 meters per second. how much energy does she lose to friction
Answers: 2
You know the right answer?
Two equilibrium reactions of nitrogen with oxygen, with their corresponding equilibrium constants (K...
Questions

Mathematics, 24.11.2020 23:20

English, 24.11.2020 23:20

Chemistry, 24.11.2020 23:20

Mathematics, 24.11.2020 23:20

English, 24.11.2020 23:20

Mathematics, 24.11.2020 23:20

History, 24.11.2020 23:20

Health, 24.11.2020 23:20

Mathematics, 24.11.2020 23:20

Mathematics, 24.11.2020 23:20

Physics, 24.11.2020 23:20


Health, 24.11.2020 23:20

English, 24.11.2020 23:20

Mathematics, 24.11.2020 23:20


English, 24.11.2020 23:20

Mathematics, 24.11.2020 23:20

Social Studies, 24.11.2020 23:20

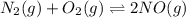
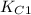 of this reaction is as follows.
of this reaction is as follows.![\frac{[NO]^{2}}{[N_{2}][O_{2}]}](/tpl/images/0541/2546/adeca.png)
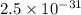
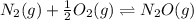
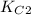 of this reaction is as follows.
of this reaction is as follows.![\frac{[N_{2}O]}{[N_{2}][O_{2}]^{\frac{1}{2}}}](/tpl/images/0541/2546/7b620.png)
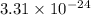
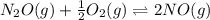
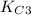 of this reaction is as follows.
of this reaction is as follows.![\frac{[NO]^{2}}{[N_{2}O][O_{2}]^{\frac{1}{2}}}](/tpl/images/0541/2546/c0a44.png)
![\frac{[NO]^{2}}{[N_{2}][O_{2}]} \times \frac{[N_{2}][O_{2}]}{[N_{2}O][O_{2}]^{\frac{1}{2}}}](/tpl/images/0541/2546/93fb5.png)
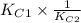
![[tex]2.5 \times 10^{-31} \times \frac{1}{3.31 \times 10^{-24}}](/tpl/images/0541/2546/55cbe.png)