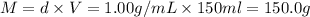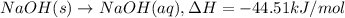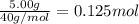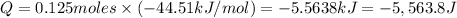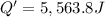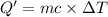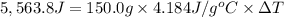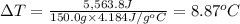Chemistry, 10.03.2020 19:01 gooberthebear8955
The enthalpy of solution (dissolving) of sodium hydroxide is given below. Determine the change in temperature of a coffee cup calorimeter containing 150 ml of water when 5.00 g NaOH (40.00 g/mol) is added to the container. You may assume that the solution has the same specific heat and density as water.
NaOH(s) → NaOH(aq) ΔH =-44.51 KJ
a. +8.87°C
b. +2.70 C
c. 2.70°C
d. 8.87°C

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 00:00
Select the correct answer. which statement is true about a polarized object? o a. it gains electrons and becomes negatively charged. ob. it gains protons and becomes positively charged. oc. the number of positive and negative charges can be the same. od. it has to be a metal. o e. there is no change in the distribution of the charge in the object. reset next what
Answers: 3

Chemistry, 22.06.2019 05:30
Liv sheldon given the balanced equation for an organic reaction: c2h2 + 2cl2 → c2h2cl4 this reaction is best classified as *
Answers: 1

Chemistry, 22.06.2019 19:00
Which change to the system wood cause the freely-moving piston to lower?
Answers: 1

Chemistry, 22.06.2019 20:30
Select all the correct answers.which compounds have the empirical formula ch20? (multiple answers)a.c2h4o2b.c3h603c.ch2o2d.c5h1005e.c6h1206
Answers: 2
You know the right answer?
The enthalpy of solution (dissolving) of sodium hydroxide is given below. Determine the change in te...
Questions

History, 30.10.2021 19:20





Chemistry, 30.10.2021 19:20

Spanish, 30.10.2021 19:20


History, 30.10.2021 19:20

World Languages, 30.10.2021 19:20

Mathematics, 30.10.2021 19:20


Social Studies, 30.10.2021 19:20



Business, 30.10.2021 19:20




English, 30.10.2021 19:20