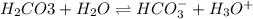Chemistry, 10.03.2020 20:00 sierranicasio
Which one would be expected to happen? If one of the buffers that contribute to pH stability in human blood is carbonic acid (H2CO3). Carbonic acid is a weak acid that when placed in an aqueous solution dissociates into a bicarbonate ion (HCO3-) and a hydrogen ion (H+). Thus, H2CO3 HCO3- + H+ If the pH of the blood increases.
A. A decrease in the concentration of HC03- and an increase in the concentration of both H2CO3 and H2O
B. An increase in the concentration of H2CO3 and a decrease in the concentration of H2O
C. An increase in the concentration of HCO3- and a decrease in the concentration of H2O
D. A decrease in the concentration of H2CO3 and an increase in the concentration of H2O
E. A decrease in the concentration of HCO3- and an increase in the concentration of H2O

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 06:00
If you burn 10 kilograms of wood in a fire (combustion) what is the weight of the products after the fire has finished burning the wood?
Answers: 3

Chemistry, 22.06.2019 09:30
In apex! a liquid heated beyond a certain temperature becomes
Answers: 1

Chemistry, 22.06.2019 12:00
A5.000 g sample of niso4 h2o decomposed to give 2.755 g of anhydrous niso4. what is the formula of the hydrate? what is the full chemical name for the hydrate? what is the molar mass of the hydrate? niso4•_h2o what is the mass % of water in the hydrate?
Answers: 1

Chemistry, 22.06.2019 14:30
Need ! asap will mark 10 pts using the room temperature line (orange line) and your periodic table, make lists that identify the state of matter (gas, liquid, or solid) in which each element you plotted exists at room temperature. explain your answers
Answers: 1
You know the right answer?
Which one would be expected to happen? If one of the buffers that contribute to pH stability in huma...
Questions


Mathematics, 22.03.2021 18:10


Mathematics, 22.03.2021 18:10




Mathematics, 22.03.2021 18:10


History, 22.03.2021 18:10


Mathematics, 22.03.2021 18:10

Mathematics, 22.03.2021 18:10


English, 22.03.2021 18:10


English, 22.03.2021 18:10