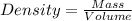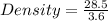Chemistry, 10.03.2020 19:21 gwendallinesikes
A 28.5 gram piece of iron is added to a graduated cylinder containing 45.5 mL of water. The water in the cylinder rises to the 49.1 mark. Calculate the density of the iron piece.

Answers: 2


Another question on Chemistry

Chemistry, 23.06.2019 07:00
Why do the strengths of london (dispersion) forces generally increase with increasing molecular size? choose one: a. heavier atoms have stronger attractions for each other than lighter atoms. b. dispersion forces are all equal in magnitude; there is no size dependence. c. dispersion forces arise from the attraction between the nuclei of atoms, and larger molecules have larger nuclei. d. dispersion forces arise from dipoles caused by the electron distribution being distorted. larger molecules have more electrons and, therefore, more distortions and a bigger force. e. dispersion forces depend on distance. larger molecules are farther apart and so the forces are smaller.
Answers: 2

Chemistry, 23.06.2019 07:30
Chris is about to do an experiment to measure the density of water at several temperatures. his teacher has him prepare and sign a safety contract before beginning the experiment. which term is mostlikely part of the safety contract
Answers: 3


Chemistry, 23.06.2019 18:10
Which property of a substance can be determined using a ph indicat
Answers: 3
You know the right answer?
A 28.5 gram piece of iron is added to a graduated cylinder containing 45.5 mL of water. The water in...
Questions

Chemistry, 26.02.2021 01:10

Mathematics, 26.02.2021 01:10

Mathematics, 26.02.2021 01:10



Social Studies, 26.02.2021 01:10

Physics, 26.02.2021 01:10




Mathematics, 26.02.2021 01:10


Mathematics, 26.02.2021 01:10





Social Studies, 26.02.2021 01:10


Mathematics, 26.02.2021 01:10