
Chemistry, 10.03.2020 22:40 sjeueujs2067
A chemist titrates 120.0 mL of a 0.4006 M hydrocyanic acid (HCN) solution with 0.6812 MNaOH solution at 25 °C. Calculate the pH at equivalence. The pKa of hydrocyanic acid is 9.21. Round your answer to 2 decimal places. Note for advanced students: you may assume the total volume of the solution equals the initial volume plus the volume of NaOH solution added

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 09:30
One way that radioactive waste is treated is by burying it in repositories. the repositories are found only in states with very low populations. true or false? a. trueb. false(also i meant to put high school but it put down middle school instead)
Answers: 1

Chemistry, 22.06.2019 13:30
An animal cell loses the ability to convert energy stored in food to energy that the cell can use. which of the cell's organelles has stopped working? a.the mitochondria b.the nucleus c.the vacuoles d.the endoplasmic reticulum
Answers: 1

Chemistry, 22.06.2019 23:00
Which organism develops breathing organism develops breathing organs from pharyngeal arches? shark, spider, sea star, sea horse
Answers: 2

Chemistry, 22.06.2019 23:00
What is the solubility-product constant of barium sulfate, baso4, if a saturated solution is 1.03 ´ 10-5 m?
Answers: 3
You know the right answer?
A chemist titrates 120.0 mL of a 0.4006 M hydrocyanic acid (HCN) solution with 0.6812 MNaOH solution...
Questions



Physics, 12.10.2020 22:01


Spanish, 12.10.2020 22:01


Mathematics, 12.10.2020 22:01



Mathematics, 12.10.2020 22:01


Mathematics, 12.10.2020 22:01

Mathematics, 12.10.2020 22:01

Mathematics, 12.10.2020 22:01

Mathematics, 12.10.2020 22:01



Social Studies, 12.10.2020 22:01


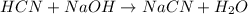
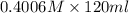
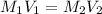
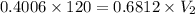
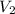 = 70.57 ml
= 70.57 ml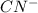 is as follows.
is as follows.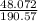
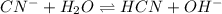
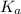 is as follows.
is as follows.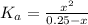
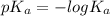
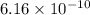
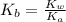
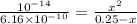
![0.2 \times 10^{-2} = [OH^{-}]](/tpl/images/0541/6496/016f5.png)
![-log[OH^{-}]](/tpl/images/0541/6496/04732.png)


