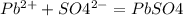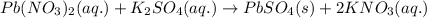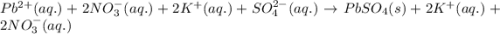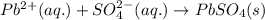Chemistry, 29.08.2019 00:00 JOEFRESH10
Lead ions can be removed from solution by precipitation with sulfate ions. suppose a solution contains lead(ii) nitrate. write a complete ionic equation to show the reaction of aqueous lead(ii) nitrate with aqueous potassium sulfate to form solid lead(ii) sulfate and aqueous potassium nitrate.

Answers: 1


Another question on Chemistry


Chemistry, 22.06.2019 12:30
Acontrol during an experiment. might change remains constant does not exist does change
Answers: 1

Chemistry, 22.06.2019 22:30
The vapor pressure of ethanol is 1.00 × 102 mmhg at 34.90°c. what is its vapor pressure at 61.61°c? (δhvap for ethanol is 39.3 kj/mol.)
Answers: 2

Chemistry, 22.06.2019 23:10
Match the formula for the following compound: magnesium sulfate heptahydratemgs·7h2omg2so4·7h2omg(so4)2·7h2omgso4·7h2o
Answers: 1
You know the right answer?
Lead ions can be removed from solution by precipitation with sulfate ions. suppose a solution contai...
Questions

Mathematics, 06.11.2020 01:00

Mathematics, 06.11.2020 01:00


Advanced Placement (AP), 06.11.2020 01:00

Mathematics, 06.11.2020 01:00

Mathematics, 06.11.2020 01:00


History, 06.11.2020 01:00

Mathematics, 06.11.2020 01:00

English, 06.11.2020 01:00

Spanish, 06.11.2020 01:00

Biology, 06.11.2020 01:00

Mathematics, 06.11.2020 01:00


Mathematics, 06.11.2020 01:00




Mathematics, 06.11.2020 01:00

Health, 06.11.2020 01:00