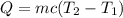Chemistry, 11.03.2020 02:11 zacharycheyne
The heat of solution is found by adding a salt to water in a calorimeter and measuring the temperature change. The specific heat of water is 4.180 Joules per g per ºC. In the calculation of the heat of solution, ignore the contribution to specific heat and mass due to the salt. Assume that these contributions are negligible. The data collected are as follows:
Grams of water in the calorimeter 46.52
Grams of salt 4.5069
Initial temperature of water 22.83 ºC
Final Temperature 18.98 ºC
Calculate the following Heat of the solution of salt.

Answers: 3


Another question on Chemistry


Chemistry, 22.06.2019 22:20
Asuspension of yeast cells is being grown under anaerobic conditions such that glucose is degraded to ethanol and carbon dioxide. if one wishes to follow this process by monitoring the release of 14co2, at which positions in the glucose molecule would the 14c label need to be incorporated?
Answers: 2


You know the right answer?
The heat of solution is found by adding a salt to water in a calorimeter and measuring the temperatu...
Questions

Physics, 31.08.2019 16:00





Physics, 31.08.2019 16:00

English, 31.08.2019 16:00


History, 31.08.2019 16:00




History, 31.08.2019 16:00




Physics, 31.08.2019 16:00