Chemistry, 11.03.2020 02:34 KillerSteamcar
Consider a carbon atom that is sp hybridized. Indicate how many of each orbital exist on this carbon atom by sorting each orbital type. Consider the outer valence only.
Options include: sp orbitals, p orbitals, s orbitals
Put them in the following categories:
Zero One Two Three

Answers: 2


Another question on Chemistry



Chemistry, 23.06.2019 04:40
Temperature is defined as a. the equivalent of heat. b. a measure of the average kinetic energy of the individual atoms or molecules composing a substance. c. how hot or cold it is. d. the total kinetic energy of the atoms or molecules composing a substance. e. none of the above is correct.
Answers: 1

Chemistry, 23.06.2019 07:30
Type the letter that represents the correct location for each particle type below. the neutron is found at __ the electron is found at __ the proton is found at __
Answers: 1
You know the right answer?
Consider a carbon atom that is sp hybridized. Indicate how many of each orbital exist on this carbon...
Questions



Health, 23.01.2020 21:31

English, 23.01.2020 21:31

Social Studies, 23.01.2020 21:31

Biology, 23.01.2020 21:31


Biology, 23.01.2020 21:31

Mathematics, 23.01.2020 21:31

Mathematics, 23.01.2020 21:31


Computers and Technology, 23.01.2020 21:31

English, 23.01.2020 21:31

Social Studies, 23.01.2020 21:31


Biology, 23.01.2020 21:31

Mathematics, 23.01.2020 21:31


Social Studies, 23.01.2020 21:31

Mathematics, 23.01.2020 21:31

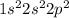 . This means that in its neutral state it contains 2 electrons in its s-orbital and 2 electrons in its p-orbital.
. This means that in its neutral state it contains 2 electrons in its s-orbital and 2 electrons in its p-orbital.