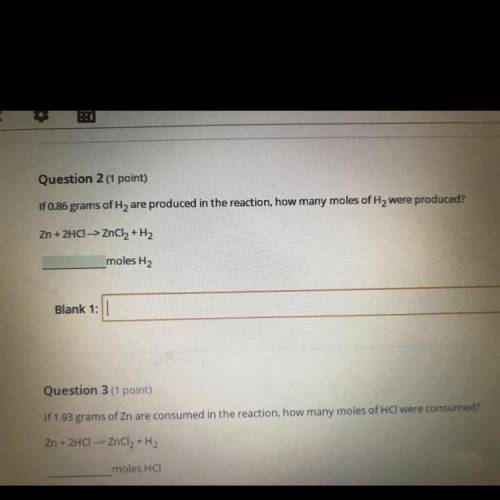
Question 2 (1 point)
If 0.86 grams of Hy are produced in the reaction, how many moles of H2 we...


Answers: 1


Another question on Chemistry

Chemistry, 21.06.2019 22:00
Which solution is an example of a nonelectrolyte? a. iodine in hexane b. sodium nitrate in waterc. acetic acid in waterd. hydrogen chloride in water
Answers: 2


Chemistry, 22.06.2019 04:30
How many moles of air are there in a human lung with a volume of 2.4 l at stp? explain your answer
Answers: 1

Chemistry, 22.06.2019 05:30
Compare and contrast physical changes with chemical changes.
Answers: 1
You know the right answer?
Questions


Mathematics, 29.01.2021 01:10


Advanced Placement (AP), 29.01.2021 01:10




Mathematics, 29.01.2021 01:10

Mathematics, 29.01.2021 01:10

Social Studies, 29.01.2021 01:10



Social Studies, 29.01.2021 01:10


Mathematics, 29.01.2021 01:10



SAT, 29.01.2021 01:10

History, 29.01.2021 01:10

Mathematics, 29.01.2021 01:10