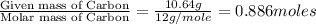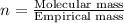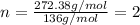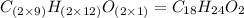Chemistry, 11.03.2020 03:31 choudharykaran7997
Combustion analysis of a 13.42-g sample of the unknown organic compound (which contains only carbon, hydrogen, and oxygen) produced 39.01 g CO2 and
10.65 g H2O. The molar mass of the unknown compound is 272.38 g/mol.
Find the molecular formula of the unknown compound.

Answers: 2


Another question on Chemistry

Chemistry, 21.06.2019 15:00
Litmus paper is made from water-soluble dyes which are extracted from lichens. this paper is used as an acid-base indicator. which of these common household substances would turn blue litmus paper red? a) bleach b) lye c) soap d) vinegar
Answers: 3


Chemistry, 23.06.2019 03:50
Show how to convert the temperature 84.7° c to kelvin. include all steps and label the final answer.
Answers: 1

Chemistry, 23.06.2019 05:00
Scientists discovered fossils in several layers of the earth you see here. they found fossils of algae, snails, and clams in layer d. given that information, where do you think they found fossil evidence of simple land plants and amphibians?
Answers: 1
You know the right answer?
Combustion analysis of a 13.42-g sample of the unknown organic compound (which contains only carbon,...
Questions




Computers and Technology, 07.06.2020 01:01





Mathematics, 07.06.2020 01:01











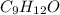 and
and 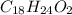
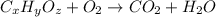

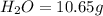
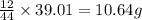 of carbon will be contained.
of carbon will be contained.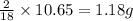 of hydrogen will be contained.
of hydrogen will be contained.