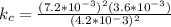A sample of pure NO2NO2 is heated to 335 ∘C∘C at which temperature it partially dissociates according to the equation 2NO2(g)⇌2NO(g)+O2(g)2NO2(g)⇌2NO(g)+ O2(g) At equilibrium the density of the gas mixture is 0.525 g/Lg/L at 0.750 atmatm .Calculate Kc for the reaction

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 05:30
Describe the interaction that occurs between two objects with the same electrical charge.
Answers: 1

Chemistry, 22.06.2019 09:30
What are scientists who study fossils called? ( a ) astronomers. ( b ) biologists. ( c ) geologists. ( d ) paleontologists.
Answers: 2

Chemistry, 22.06.2019 12:00
What does a complete balanced chemical equation include? a. exothermic coefficients b. endothermic coefficients c. valence electrons d. molar coefficients
Answers: 1

Chemistry, 22.06.2019 14:50
Which of the following is most likely true about water in chemical solutions?
Answers: 1
You know the right answer?
A sample of pure NO2NO2 is heated to 335 ∘C∘C at which temperature it partially dissociates accordin...
Questions

















Geography, 13.09.2019 03:10

Mathematics, 13.09.2019 03:10


Mathematics, 13.09.2019 03:10

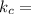 1. 1 × 10⁻²
1. 1 × 10⁻²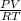
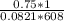
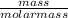
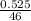
![k_c = \frac{[NO]^2[O_2]}{[NO_2]^2}](/tpl/images/0542/3911/d520a.png)