
Chemistry, 11.03.2020 04:33 ashleyjohnson2002
(a) Write the dissolution reaction for solid Na2CO3 below. (Use the lowest possible coefficients. Include states-of-matter under the given conditions in your answer.)
(b) Once the ionic solid has dissolved, the anion that is formed is able to react as a base, with water as the acid. Write the net acid-base reaction that occurs when dissolved Na2CO3 reacts with water. (Use the lowest possible coefficients. Omit states-of-matter in your answer.)

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 10:00
How many grams of co(g) are there in 74.5 ml of the gas at 0.933 atm and 30o c?
Answers: 1

Chemistry, 22.06.2019 11:30
If blood contains 150g of hemoglobin per liter of blood, how much hemoglobin would be contained in 10 ml of blood
Answers: 2


Chemistry, 22.06.2019 22:00
In order to complete this lab. you will need to be familiar with some common chemistry terms. complete the chemical change puzzle and list the relevant terms and their meaning below a.rectant b.product c.supernate
Answers: 3
You know the right answer?
(a) Write the dissolution reaction for solid Na2CO3 below. (Use the lowest possible coefficients. In...
Questions



Mathematics, 26.12.2019 15:31


History, 26.12.2019 15:31


Mathematics, 26.12.2019 15:31

Mathematics, 26.12.2019 15:31


Mathematics, 26.12.2019 15:31


Mathematics, 26.12.2019 15:31

Business, 26.12.2019 15:31


Mathematics, 26.12.2019 15:31

Health, 26.12.2019 15:31

Business, 26.12.2019 15:31

Business, 26.12.2019 15:31

Physics, 26.12.2019 15:31

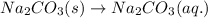
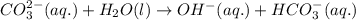
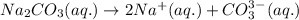
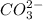 reacts with water to form conjugate acid.
reacts with water to form conjugate acid.

