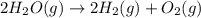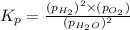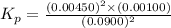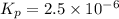Answers: 3


Another question on Chemistry


Chemistry, 22.06.2019 14:50
Which of the following is most likely true about water in chemical systems? a) water dissolves nonpolar ionic compounds. b) water dissociates ionic compounds. c) water dissociates covalent molecules. d) water dissolves nonpolar covalent substances.
Answers: 1

Chemistry, 22.06.2019 22:30
Write and balance the chemical equation that represents the reaction of aqueous sulfuric acid with aqueous sodium hydroxide to form water and sodium sulfate. include phases.
Answers: 1

Chemistry, 23.06.2019 00:50
Which of the following warnings would an agricultural chemist tell a farmer who wants to recycle his or her own ammonia? recycling ammonia is a difficult process that sometimes takes weeks. recycling ammonia requires a degree in biochemistry or a related field. recycling ammonia can be harmful because it is highly flammable and toxic. recycling ammonia costs too much money considering the price of the necessary chemicals.
Answers: 1
You know the right answer?
The elementary reaction 2H20(g)<--->2H2(g)+O2(g) proceeds at a certain temperature until the p...
Questions








Physics, 15.10.2020 09:01



Mathematics, 15.10.2020 09:01

Physics, 15.10.2020 09:01

Mathematics, 15.10.2020 09:01

Mathematics, 15.10.2020 09:01

History, 15.10.2020 09:01

Mathematics, 15.10.2020 09:01




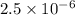
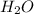 = 0.0900 atm
= 0.0900 atm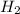 = 0.00450 atm
= 0.00450 atm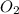 = 0.00100 atm
= 0.00100 atm