
Chemistry, 11.03.2020 05:07 shacarabrown49
Consider four different samples: aqueous LiBr, molten LiBr, aqueous AgBr, and molten AgBr. Current run through each sample produces one of the following products at the cathode: solid lithium, solid silver, or hydrogen gas. Match each sample to its cathodic product. Drag the appropriate items to their respective bins.

Answers: 2


Another question on Chemistry

Chemistry, 21.06.2019 17:00
What were the success and failures that came to boyle’s excitements?
Answers: 1

Chemistry, 21.06.2019 20:20
Calculate the molarity of the solution. 6.02 x 1022 molecules of hci (molecular weight = 36.5 g/mole) in 2.0 liters of water m
Answers: 1

Chemistry, 22.06.2019 01:30
(apex) when a cup of water is dropped, as the cup falls, the water in the cup falls out true or false?
Answers: 1

Chemistry, 22.06.2019 08:30
Agroup of students is studying convection current. they fill two identical balloons with the same amount of helium. one balloon is placed in a freezer and the other is in an area with warm air. after 10 minutes, the balloon are released from a height of 1 meter. which of the following to the students most likely observe? a) the warm balloon expands and rises. the cold balloon shrinks and sinks b) the balloon both rise. the cold balloon is larger than the warm balloon c) the cold balloon expands and rises. the warm balloon shrinks and sinks d) the balloon rise at the same rate. both balloons are the same size
Answers: 1
You know the right answer?
Consider four different samples: aqueous LiBr, molten LiBr, aqueous AgBr, and molten AgBr. Current r...
Questions




Mathematics, 14.07.2019 19:00

Mathematics, 14.07.2019 19:00

Mathematics, 14.07.2019 19:00

Spanish, 14.07.2019 19:00



Mathematics, 14.07.2019 19:00





Mathematics, 14.07.2019 19:00


Mathematics, 14.07.2019 19:00


Mathematics, 14.07.2019 19:00

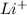 (aq) +
(aq) +  → Li(s)
→ Li(s)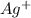 (aq) +
(aq) + 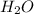 ions and LiBr ions compete with each other. Since H+ has a greater standard redox potential than lithium,H+ will be reduced at the cathode to produce hydrogen gas
ions and LiBr ions compete with each other. Since H+ has a greater standard redox potential than lithium,H+ will be reduced at the cathode to produce hydrogen gas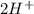 (aq) +
(aq) + 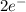 →
→ 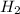 (g)
(g)

