
Chemistry, 11.03.2020 06:06 QuestionsAnsweredNow
A mixture of CO2 and Kr weighs 41.0 g and exerts a pressure of 0.729 atm in its container. Since Kr is expensive, you wish to recover it from the mixture. After the CO2 is completely removed by absorption with NaOH(s), the pressure in the container is 0.193 atm. (a) How many grams of CO2 were originally present? (b) How many grams of Kr can you recover?

Answers: 3


Another question on Chemistry

Chemistry, 21.06.2019 19:00
State the formula for density in words and mathematical symbols
Answers: 2

Chemistry, 22.06.2019 12:30
Which element has the lowest electronegativity? calcium(ca) gallium(ga) selenium(se) bromine(br)
Answers: 1

Chemistry, 22.06.2019 14:00
What mass of natural gas (ch4) must you burn to emit 276 kj of heat?
Answers: 1

Chemistry, 22.06.2019 20:30
Water undergoes a large change in density at 0 ∘ c as it freezes to form ice. calculate the percent change in density that occurs when liquid water freezes to ice at 0 ∘ c given that
Answers: 2
You know the right answer?
A mixture of CO2 and Kr weighs 41.0 g and exerts a pressure of 0.729 atm in its container. Since Kr...
Questions

Mathematics, 17.12.2020 20:00



Mathematics, 17.12.2020 20:00

Mathematics, 17.12.2020 20:00

Mathematics, 17.12.2020 20:00


Mathematics, 17.12.2020 20:00

History, 17.12.2020 20:00


Mathematics, 17.12.2020 20:00


Biology, 17.12.2020 20:00

Physics, 17.12.2020 20:00

Mathematics, 17.12.2020 20:00

Mathematics, 17.12.2020 20:00



Mathematics, 17.12.2020 20:00

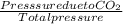
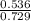
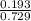
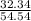 * 100 %
* 100 % 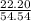 * 100 %
* 100 % 


