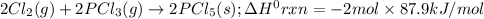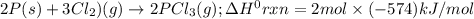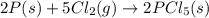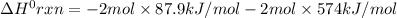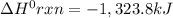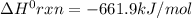Chemistry, 11.03.2020 06:31 cyanezc1313
Consider the following thermochemical equations. PCl5 (s)→PCl3 (g)+Cl2 (g)2P (s)+3Cl2 (g)→2PCl3 (g)ΔH∘rxn=87.9kJmol ΔH∘rxn=−574kJmol Using this data, determine the heat of formation for PCl5.

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 00:40
Base your answer on the information below and on your knowledge of chemistry. nitrogen dioxide, no2, is a dark brown gas that is used to make nitric acid and to bleach flour. nitrogen dioxide has a boiling point of 294 k at 101.3 kpa. in a rigid cylinder with a movable piston, nitrogen dioxide can be in equilibrium with colorless dinitrogen tetroxide, n2o4. this equilibrium is represented by the equation below. 2no2(g) n2o4(g) + 58kj at standard pressure, compare the strength of intermolecular forces in no2(g) to the strength of intermolecular forces in n2(g).
Answers: 2

Chemistry, 22.06.2019 01:30
(apex) when a cup of water is dropped, as the cup falls, the water in the cup falls out true or false?
Answers: 1

Chemistry, 22.06.2019 08:00
An electron moved from shell n = 2 to shell n = 1. what most likely happened during the transition? a fraction of a photon was added. a photon of energy was absorbed. a fraction of a photon was removed. a photon of energy was released.
Answers: 1

Chemistry, 22.06.2019 18:30
When a device is used in a circuit in which the voltage is 81 v the current flowing through the device is 3 a what is the resistance of the device
Answers: 2
You know the right answer?
Consider the following thermochemical equations. PCl5 (s)→PCl3 (g)+Cl2 (g)2P (s)+3Cl2 (g)→2PCl3 (g)Δ...
Questions




History, 16.11.2019 05:31





Biology, 16.11.2019 05:31





Chemistry, 16.11.2019 05:31



Mathematics, 16.11.2019 05:31