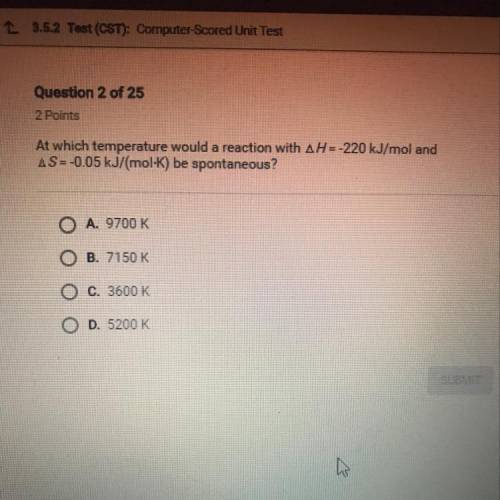
At which temperature would a reaction with AH = -220 kJ/mol and
AS=-0.05 kJ/(mol-K) be spontan...

Chemistry, 11.03.2020 09:51 michaelchavez6959127
At which temperature would a reaction with AH = -220 kJ/mol and
AS=-0.05 kJ/(mol-K) be spontaneous?

Answers: 3


Another question on Chemistry

Chemistry, 21.06.2019 18:30
In an oxidation-reduction reaction, oxidation is what happens when a reactant
Answers: 1

Chemistry, 21.06.2019 21:00
Of the groups of elements below, which are most likely to gain electrons to become anions? a. alkali metal b. boron group c. halogen d. transition metal
Answers: 2

Chemistry, 21.06.2019 22:50
Blank allows you to do calculations for situations in which only the amount of gas is constant a)boyle's law b)combined gas law c)ideal gas law d)dalton's law
Answers: 1

You know the right answer?
Questions

History, 25.11.2021 06:40



Computers and Technology, 25.11.2021 06:40






English, 25.11.2021 06:40

Social Studies, 25.11.2021 06:40

Mathematics, 25.11.2021 06:40


Mathematics, 25.11.2021 06:40


Mathematics, 25.11.2021 06:40

Biology, 25.11.2021 06:40






