Chemistry, 11.03.2020 17:09 sylaspotter707
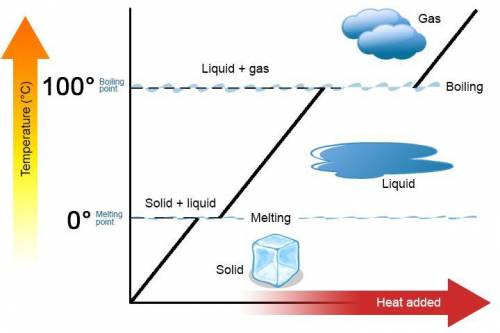
The specific heat of liquid ethanol, C2H5OH(l) is 2.46j/g degree celsius and the heat of vaporization is 39.3 kj/mol the boiling point of ethanol is 78.3 c what amount of etnthalpy is required to heat 50 g of liquid ethanol form 23 c to ethanol vapor a 78.3 c?

Answers: 3


Another question on Chemistry

Chemistry, 21.06.2019 15:50
Where are chemicals found at work? a. only in cleaning products b. only in carpets and paint c. in every area of work d. only in food preparation submit
Answers: 1

Chemistry, 21.06.2019 21:30
The density of an unknown gas at 98°c and 740 mmhg is 2.50 g/l. what is the molar mass of the gas with work showed?
Answers: 1


Chemistry, 22.06.2019 03:30
Each pair of clay balls represents to planetesimals if each plane test molluscum pound of the same material and is separated by the same distance which pair experiences the greatest gravitational attraction
Answers: 2
You know the right answer?
The specific heat of liquid ethanol, C2H5OH(l) is 2.46j/g degree celsius and the heat of vaporizatio...
Questions

Mathematics, 08.09.2019 07:10

Biology, 08.09.2019 07:10




Mathematics, 08.09.2019 08:10

History, 08.09.2019 08:10




Mathematics, 08.09.2019 08:10

Health, 08.09.2019 08:10





Mathematics, 08.09.2019 08:10

History, 08.09.2019 08:10


Mathematics, 08.09.2019 08:10

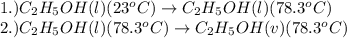
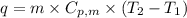
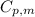 = specific heat capacity of liquid ethanol = 2.46 J/g°C
= specific heat capacity of liquid ethanol = 2.46 J/g°C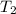 = final temperature = 78.3°C
= final temperature = 78.3°C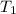 = initial temperature = 23°C
= initial temperature = 23°C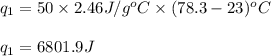
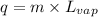
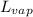 = latent heat of vaporization = 39.3 kJ/mol
= latent heat of vaporization = 39.3 kJ/mol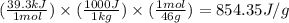
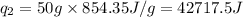
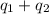
![[6801.9+42717.5]J=49519.4J=49.52kJ](/tpl/images/0542/9395/07659.png)