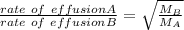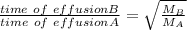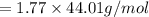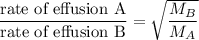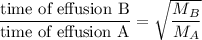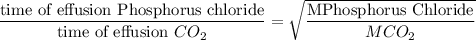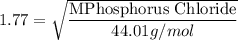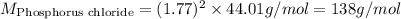Chemistry, 11.03.2020 16:55 FantasticFerret
When 2.66 g of phosphorus was burned in chlorine, the product was a phosphorus chloride. Its vapor took 1.77 times as long to effuse as the same amount of CO2 under the same conditions of temperature and pressure. What is the molar mass of the phosphorus chloride? 1. 138 g/mol 2. 102 g/mol 3. 156 g/mol 4. 87.7 g/mol

Answers: 1


Another question on Chemistry


Chemistry, 23.06.2019 11:00
The image below shows a weather service map.. i’m not sure if is correct
Answers: 2

Chemistry, 23.06.2019 16:00
Henry moseley used x-ray experiments to determine the atomic number of elements. how did his discovery contribute to the development of the periodic table? a.it confirmed that elements should be arranged in strict order of increasing atomic mass. b.it led to elements with similar atomic numbers being grouped together. c.it allowed the elements to be placed in strict order of increasing atomic number. d.it showed that the way mendeleev grouped elements together was completely wrong.
Answers: 1

Chemistry, 23.06.2019 21:10
For the reaction shown compute the theoretical yield of product in moles each of the initial quantities of reactants. 2 mn(s)+3 o2 (g) mno2(s) 2 mol mn , 2 mol o2
Answers: 1
You know the right answer?
When 2.66 g of phosphorus was burned in chlorine, the product was a phosphorus chloride. Its vapor t...
Questions

Chemistry, 21.05.2021 05:00

Geography, 21.05.2021 05:00

Mathematics, 21.05.2021 05:00


Mathematics, 21.05.2021 05:00



English, 21.05.2021 05:00


History, 21.05.2021 05:00


Biology, 21.05.2021 05:00

English, 21.05.2021 05:00

Mathematics, 21.05.2021 05:00


Mathematics, 21.05.2021 05:00