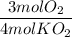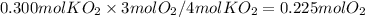Chemistry, 11.03.2020 17:25 gadgetady5699
The reaction between potassium superoxide, KO₂, and CO₂,
is used as a source of O₂ and absorber of CO₂ in self-contained breathing equipment used by rescue workers.
How many moles of O₂ are produced when 0.300 mol of KO₂ reacts in this fashion?

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 03:00
Flourine is found to undergo 10% radioactivity decay in 366 minutes determine its halflife
Answers: 3

Chemistry, 22.06.2019 04:30
Which statement best describes the relationship between period and frequency of light waves? a) in wave b the period increases and the frequency decreases from wave a. b) in wave a the period increases and the frequency decreases from wave b. c) in wave b the period is shorter and the frequency is greater than in wave a. d) in wave a the period is shorter and the frequency is greater than in wave b.
Answers: 1

Chemistry, 22.06.2019 07:00
This image is an example of a(n) a) atom. b) compound. c) mixture. d) molecule.
Answers: 1

Chemistry, 22.06.2019 08:00
This classification of drug typically changes the brain's chemistry and reduces its ability to create its own endorphins.
Answers: 1
You know the right answer?
The reaction between potassium superoxide, KO₂, and CO₂,
is used as a source of O₂ and...
is used as a source of O₂ and...
Questions

Mathematics, 22.02.2021 09:00


Mathematics, 22.02.2021 09:00

Mathematics, 22.02.2021 09:00

Mathematics, 22.02.2021 09:00

Physics, 22.02.2021 09:00


Mathematics, 22.02.2021 09:00

History, 22.02.2021 09:00

Mathematics, 22.02.2021 09:00

Health, 22.02.2021 09:00

Arts, 22.02.2021 09:00

Spanish, 22.02.2021 09:00

Mathematics, 22.02.2021 09:00

Advanced Placement (AP), 22.02.2021 09:00


History, 22.02.2021 09:00

Mathematics, 22.02.2021 09:00


Mathematics, 22.02.2021 09:00