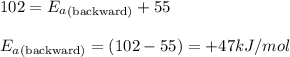Chemistry, 11.03.2020 19:05 lizbethh62
The decomposition of dinitrogen pentaoxide has an activation energy of 102 kJ/mol and ΔH°rxn = + 55 kJ/mol. What is the activation energy for the reverse reaction?

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 01:40
Brainliesttt me asap! the below reaction can be categorized as more than one type of reaction. which reactions are these, and what are the types of reactions?
Answers: 1

Chemistry, 22.06.2019 03:10
Agas diffuses 1/7 times faster than hydrogen gas (h2). what is the molar mass of the gas? 100.10 g/mol 98.78 g/mol 86.68 g/mol 79.98 g/mol
Answers: 3

Chemistry, 22.06.2019 04:40
In which environment would primary succession occur? a forest with a few remaining trees after a recent wildfire an area of exposed rock after a glacier melts away beach that is exposed to the air at low tide an abandoned baseball field in a small town
Answers: 1

You know the right answer?
The decomposition of dinitrogen pentaoxide has an activation energy of 102 kJ/mol and ΔH°rxn = + 55...
Questions



Biology, 01.01.2020 20:31

Chemistry, 01.01.2020 20:31






Mathematics, 01.01.2020 20:31



Mathematics, 01.01.2020 20:31



Spanish, 01.01.2020 20:31

Mathematics, 01.01.2020 20:31


Mathematics, 01.01.2020 20:31

English, 01.01.2020 20:31

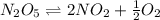
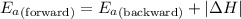
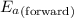 = Activation energy of the forward reaction = 102 kJ/mol
= Activation energy of the forward reaction = 102 kJ/mol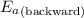 = Activation energy of the backward reaction = ?
= Activation energy of the backward reaction = ?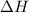 = Enthalpy of the reaction = +55 kJ/mol
= Enthalpy of the reaction = +55 kJ/mol