

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 10:50
Determine the empirical formula for succinic acid that is composed of 40.60% carbon, 5.18% hydrogen, and 54.22% oxygen.
Answers: 1


Chemistry, 23.06.2019 05:30
Idont understand it 1.what is the boiling point of a solution of 675 grams of ethylene glycol (c2h6o2) in 2.50 liters of water?
Answers: 2

Chemistry, 23.06.2019 07:00
Select the correct answer. why are scientific models important in the study of science? a. they always involve critical mathematical calculations. b. they scientists understand complex ideas and objects that aren’t easy to handle. c. they enable scientists to popularize their work in society. d. they are required when conducting any peer review process. e. they are necessary for turning a hypothesis into a law.
Answers: 2
You know the right answer?
What is the pH of a solution prepared by mixing 30.00 mL of 0.10 MCH3CO2H with 30.00 mL of 0.030 MCH...
Questions



Mathematics, 04.03.2021 21:00

Mathematics, 04.03.2021 21:00

Physics, 04.03.2021 21:00



Mathematics, 04.03.2021 21:00

Mathematics, 04.03.2021 21:00

Business, 04.03.2021 21:00

Social Studies, 04.03.2021 21:00




Business, 04.03.2021 21:00


English, 04.03.2021 21:00



English, 04.03.2021 21:00


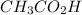 = 0.10 M
= 0.10 M = 0.030 M
= 0.030 M .
.
 in this expression, we get:
in this expression, we get:


![pH=pK_a+\log \frac{[Salt]}{[Acid]}](/tpl/images/0543/3032/e961a.png)
![pH=pK_a+\log \frac{[CH_3CO_2K]}{[CH_3CO_2H]}](/tpl/images/0543/3032/8b433.png)




