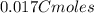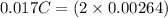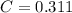Chemistry, 11.03.2020 22:15 hiiliohi1018
An aqueous solution of perchloric acid is standardized by titration with a 0.191 M solution of barium hydroxide. If 13.8 mL of base are required to neutralize 17.0 mL of the acid, what is the molarity of the perchloric acid solution?

Answers: 1


Another question on Chemistry

Chemistry, 21.06.2019 22:30
Complete the sentence. the lower the hydrogen ion concentration, the the ph. higher lower closer to 7 closer to 0
Answers: 2

Chemistry, 22.06.2019 07:10
Provide a stepwise curved arrow mechanism that fully explains the outcome of the reaction shown below. oh нао* heat он
Answers: 2

Chemistry, 22.06.2019 14:00
650.j is the same amount of energy as? 2720cal1550cal650.cal2.72cal
Answers: 2

Chemistry, 22.06.2019 15:00
Which theory was contradicted by experiments with the photoelectric effect? light spreads out after it passes through a small opening. as soon as light strikes metal, electrons will be ejected. visible light, regardless of color, will cause the ejection of electrons when striking metal. the kinetic energy of ejected electrons depends on the frequency of light that strikes the metal.
Answers: 2
You know the right answer?
An aqueous solution of perchloric acid is standardized by titration with a 0.191 M solution of bariu...
Questions

English, 27.11.2020 08:10

Mathematics, 27.11.2020 08:10




Business, 27.11.2020 08:10

Mathematics, 27.11.2020 08:10

Biology, 27.11.2020 08:10

Chemistry, 27.11.2020 08:10


Mathematics, 27.11.2020 08:10


Mathematics, 27.11.2020 08:10


SAT, 27.11.2020 08:10


English, 27.11.2020 08:10



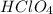 solution is 0.311 M
solution is 0.311 M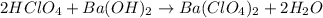
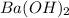 neutralizes 2 moles of
neutralizes 2 moles of 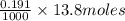 =
= 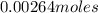
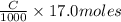 =
=