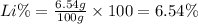Chemistry, 11.03.2020 22:01 catboy7196
Aluminum–lithium (Al–Li) alloys have been developed by the aircraft industry to reduce the weight and improve the performance of its aircraft. A commercial aircraft skin material having a density of 2.14 g/cm3 is desired. Compute the concentration of Li (in wt%) that is required. The densities of aluminum and lithium are 2.71 and 0.534 g/cm3, respectively.

Answers: 1


Another question on Chemistry

Chemistry, 21.06.2019 22:30
What are the charges of the subatomic particles by choosing the answer from the drop down menu. protons have a (+1,0,or-1). (protons,neutrons,electrons) have a 0 charge. 3.) electrons have a (+1,0,-1)
Answers: 2

Chemistry, 21.06.2019 22:30
How many moles are in 250 grams of tungsten (w)? * 4.4x10^23 moles 4.2x10^23 moles 0.7 moles 1.4 moles
Answers: 3

Chemistry, 22.06.2019 05:30
Arecipe calls for 1.2 cups of oil. how many liters of oil is this?
Answers: 2

Chemistry, 22.06.2019 22:00
Ill give u brainliest pls how is mass of carbon conserved during cellular respiration
Answers: 1
You know the right answer?
Aluminum–lithium (Al–Li) alloys have been developed by the aircraft industry to reduce the weight an...
Questions




Physics, 06.12.2021 07:50


English, 06.12.2021 07:50

History, 06.12.2021 07:50

History, 06.12.2021 07:50

Arts, 06.12.2021 07:50

History, 06.12.2021 07:50


English, 06.12.2021 07:50

Arts, 06.12.2021 07:50

Mathematics, 06.12.2021 07:50

English, 06.12.2021 07:50


Mathematics, 06.12.2021 07:50

Mathematics, 06.12.2021 07:50

Mathematics, 06.12.2021 07:50

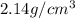
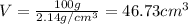
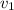
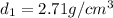
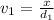
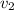
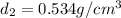
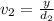
 ..[1]
..[1]
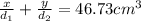
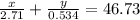 ..[2]
..[2]