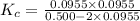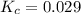Chemistry, 11.03.2020 22:49 webbjalia04
At high temperature, 2.00 mol of HBr was placed in a 4.00 L container where it decomposed in the reaction: 2HBr(g) H2(g) Br2(g) At equilibrium the concentration of Br2 was measured to be 0.0955 M. What is Kc for this reaction at this temperature

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 01:00
Look at the bean data from days 4–6. use these data to explain how natural selection changed the number of dark red walking beans over time. writing part
Answers: 3

Chemistry, 22.06.2019 03:50
Consider the reaction: n2(g) + o2(g) ? 2no(g) kc = 0.10 at 2000oc starting with initial concentrations of 0.040 mol/l of n2 and 0.040 mol/l of o2, calculate the equilibrium concentration of no in mol/l how would this be done?
Answers: 3

Chemistry, 22.06.2019 19:00
Convert the temperature of dry ice, –77 ∞c, into degrees fahrenheit and kelvin.
Answers: 2

You know the right answer?
At high temperature, 2.00 mol of HBr was placed in a 4.00 L container where it decomposed in the rea...
Questions

Mathematics, 03.02.2020 11:01



Biology, 03.02.2020 11:01

Health, 03.02.2020 11:01

History, 03.02.2020 11:01

Mathematics, 03.02.2020 11:01

Advanced Placement (AP), 03.02.2020 11:01


Mathematics, 03.02.2020 11:01



Mathematics, 03.02.2020 11:01

Biology, 03.02.2020 11:01



Mathematics, 03.02.2020 11:01


Mathematics, 03.02.2020 11:01

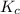 for this reaction at this temperature is 0.029
for this reaction at this temperature is 0.029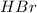 = 2.00 mole
= 2.00 mole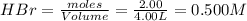
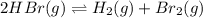
![K_c=\frac{[H_2\times [Br_2]}{[HBr]^2}](/tpl/images/0543/5528/3a3d4.png)
![[Br_2]](/tpl/images/0543/5528/f23ed.png) = x = 0.0955 M
= x = 0.0955 M