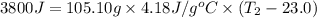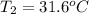Chemistry, 11.03.2020 22:58 spotty1023
In the following experiment, a coffee-cup calorimeter containing 100 mL of H2O is used. The initial temperature of the calorimeter is 23.0 ∘C. If 5.10 g of CaCl2 is added to the calorimeter, what will be the final temperature of the solution in the calorimeter? The heat of solution ΔHsoln of CaCl2 is −82.8 kJ/mol .

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 14:30
According to le chatelier’s principle, a system in chemical equilibrium responds to stress by shifting the equilibrium in a direction that reduces the stress. normalizes the stress. increases the stress. changes the stress.
Answers: 1

Chemistry, 22.06.2019 18:30
How many moles of bromine are needed to produce 3.23 moles of potassium bromide
Answers: 1


Chemistry, 23.06.2019 04:31
2ki + pb(no3)2 → 2kno3 + pbi2 determine how many moles of kno3 are created if 0.03 moles of ki are completely consumed.
Answers: 1
You know the right answer?
In the following experiment, a coffee-cup calorimeter containing 100 mL of H2O is used. The initial...
Questions




Mathematics, 26.03.2020 23:56



Mathematics, 26.03.2020 23:56





Mathematics, 26.03.2020 23:56








History, 26.03.2020 23:56

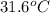
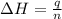
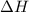 = enthalpy change = 82.8 kJ/mol
= enthalpy change = 82.8 kJ/mol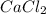 = 5.10 g
= 5.10 g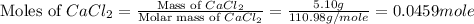
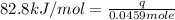
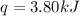
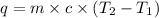
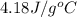
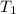 = initial temperature =
= initial temperature = 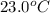
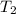 = final temperature = ?
= final temperature = ?