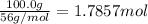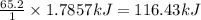Chemistry, 11.03.2020 22:59 isabellacampos4586
Calcium oxide reacts with water to produce calcium hydroxide and 65.2 kJ of heat in the following reaction. CaO (s) + H2O (l) → Ca(OH)2 (s) ΔH = - 65.2 kJ How much heat is released when 100.0 g of calcium oxide reacts with excess water?

Answers: 2


Another question on Chemistry


Chemistry, 22.06.2019 10:00
How many mmols of tris-hcl are there in 100 ml of a 100 mm tris-hcl buffer solution at ph 8.1? note that the 100 mm refers to the sum of tris and tris-hcl concentrations?
Answers: 3

Chemistry, 22.06.2019 15:30
Draw the lewis dot structure for each of the following polyatomic ions
Answers: 1

Chemistry, 22.06.2019 18:50
Asample of tin (ii) chloride has a mass of 0.49 g. after heating, it has a mass of 0.41 g. what is the percent by mass of water in the hydrate? %
Answers: 1
You know the right answer?
Calcium oxide reacts with water to produce calcium hydroxide and 65.2 kJ of heat in the following re...
Questions

Mathematics, 23.10.2020 01:01

Health, 23.10.2020 01:01



Chemistry, 23.10.2020 01:01

Mathematics, 23.10.2020 01:01



History, 23.10.2020 01:01



Mathematics, 23.10.2020 01:01


Social Studies, 23.10.2020 01:01

Mathematics, 23.10.2020 01:01


Mathematics, 23.10.2020 01:01

Biology, 23.10.2020 01:01

Health, 23.10.2020 01:01

Mathematics, 23.10.2020 01:01

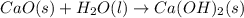 , ΔH = -65.2 kJ
, ΔH = -65.2 kJ