
Chemistry, 11.03.2020 23:39 samantha9014
An aqueous potassium iodate ( KIO3 ) solution is made by dissolving 562 562 grams of KIO 3 KIO3 in sufficient water so that the final volume of the solution is 4.30 L. Calculate the molarity of the KIO 3 KIO3 solution.

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 02:50
Consider the equilibrium system: 2icl(s) ⇄ i2(s) + cl2(g) which of the following changes will increase the total amount of of cl2 that can be produced? all of the listed answers are correct decreasing the volume of the container removing the cl2 as it is formed adding more icl(s) removing some of the i2(s)
Answers: 1

Chemistry, 23.06.2019 01:00
Which of the following is a physical change? a.burning a piece of wood b.sawing a piece of wood in half c.rust forming on an iron fence d.a copper roof changing color from orange to green
Answers: 1


You know the right answer?
An aqueous potassium iodate ( KIO3 ) solution is made by dissolving 562 562 grams of KIO 3 KIO3 in s...
Questions




Business, 27.02.2020 21:52

Mathematics, 27.02.2020 21:52






Mathematics, 27.02.2020 21:52


Mathematics, 27.02.2020 21:52

Chemistry, 27.02.2020 21:52


Biology, 27.02.2020 21:53




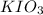 solution is 0.612 mol/L
solution is 0.612 mol/L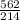 moles of
moles of 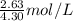 = 0.612 mol/L
= 0.612 mol/L

