
Aqueous sulfuric acid will react with solid sodium hydroxide to produce aqueous sodium sulfate and liquid water . Suppose 67.7 g of sulfuric acid is mixed with 83. g of sodium hydroxide. Calculate the minimum mass of sulfuric acid that could be left over by the chemical reaction. Round your answer to significant digits.

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 09:00
Particles vibrate in a rigid structure and do not move relative to their neighbors.
Answers: 1



Chemistry, 22.06.2019 15:30
Which of the following are correct values for the ideal gas laws constant r
Answers: 1
You know the right answer?
Aqueous sulfuric acid will react with solid sodium hydroxide to produce aqueous sodium sulfate and l...
Questions





Chemistry, 22.01.2020 21:31

Business, 22.01.2020 21:31






Biology, 22.01.2020 21:31


Mathematics, 22.01.2020 21:31

History, 22.01.2020 21:31



Mathematics, 22.01.2020 21:31

Mathematics, 22.01.2020 21:31

Social Studies, 22.01.2020 21:31




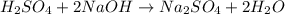
 require 2 moles of
require 2 moles of 
 of
of 

