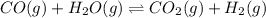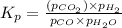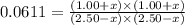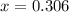Chemistry, 12.03.2020 00:02 Mitchmorgan3816
CO(g ) + H2O(g ) <---> CO2(g ) + H2(g ), Kc = 0.0611 at 2000 K . A reaction mixture initially contains a CO partial pressure of 2.50 atm, an H2O partial pressure of 2.50 atm, a CO2 partial pressure of 1.00 atm, and an H2 partial pressure of 1.00 atm at 2000 K. Calculate the equilibrium partial pressure of CO

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 02:00
The alkali metals (group 1) consist of lithium (3), sodium (11), potassium (19), rubidium (37), cesium (55), and francium (87). they are soft, metallic solids with low densities and low melting points. based on the data shown in figure 1, how many valence electrons do alkali metals share?
Answers: 3

Chemistry, 22.06.2019 06:00
If a polyatomic ionic compound has gained two hydrogen ions, then how does its name begin?
Answers: 3

Chemistry, 22.06.2019 06:00
One of the few xenon compounds that form is cesium xenon heptafluoride (csxef7). how many moles of csxef7 can be produced from the reaction of 13.0 mol cesium fluoride with 12.5 mol xenon hexafluoride? csf(s) + xef6(s) csxef7(s)
Answers: 1

Chemistry, 22.06.2019 17:20
Pegmatites are igneous rocks in which the individual minerals are very large. typically, the minerals are all light-colored quartz, feldspar and muscovite. if you were given a black and white photograph of a pegmatite in a quarry (where the rock has been blasted and broken), what physical properties could you use to identify those three minerals in this hypothetical photo? describe each mineral and the specific diagnostic properties. be specific.
Answers: 2
You know the right answer?
CO(g ) + H2O(g ) <---> CO2(g ) + H2(g ), Kc = 0.0611 at 2000 K . A reaction mixture initially...
Questions

Mathematics, 20.11.2020 21:00


Chemistry, 20.11.2020 21:00


Mathematics, 20.11.2020 21:00


Mathematics, 20.11.2020 21:00




Mathematics, 20.11.2020 21:00

Chemistry, 20.11.2020 21:00




Social Studies, 20.11.2020 21:00

History, 20.11.2020 21:00


Mathematics, 20.11.2020 21:00

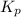 is the constant of a certain reaction at equilibrium.
is the constant of a certain reaction at equilibrium.